- Portfolio News
- 21 November, 2024

The new funding was led by Balderton Capital and Future Positive Capital.
Launched in 2013 by Ida Tin and Hans Raffauf, Clue empowers women and people with cycles to make better choices for themselves around their menstrual, sexual and reproductive health and wellbeing, starting with personalised period tracking.Since the beginning, Clue has been a trailblazer in combining science, research and data protection to provide a trusted service to its global user base. The platform now has users in over 190 countries around the world, accessing information that helps them manage their health by tracking their experience in 15 different languages through the free app. The Clue Plus paid subscription gives users access to premium features including symptom predictions, cycle analysis, Clue Pregnancy mode and curated content.

Audrey Tsang (co-CEO), Ida Tin (co-founder & chairwoman), Carrie Walter (co-CEO), Clue
At Clue, we’re focused on empowering women and people with cycles with trustworthy information and empathetic tools to better understand their bodies, make good choices for themselves, self-manage and self-advocate. To deliver on this, and specifically as we prepare to launch Clue Birth Control, we’ve had to grow as an organization to work in an even more responsible and transparent way. We’re delighted to be welcoming new investors that believe in this mission and can support and advise us as we continue to develop new features that empower women and people with cycles across the world.
Audrey Tsang CEO, Clue
Clue is a leader in femtech, a term coined by former CEO and now Chairwoman Ida Tin, to define the group of technologies that are designed to support and advance female health. With an addressable market of half the world’s population but until recently considered a “niche” area, femtech is expected to be worth $60 billion by 2027.The next step in Clue’s mission is to launch medical-grade features within the Clue app. This means that Clue is now the first mass consumer app that is also a direct-to-consumer medical device company. The first addition to this platform is Clue Birth Control, its FDA-cleared digital contraceptive, which is now being rolled out to eligible users in a limited US launch. Clue Birth Control uses tracked period start dates to allow women to monitor their fertility and prevent pregnancy. It combines the user’s menstrual cycle data with a mathematical model derived from clinical research data to predict which days they are at high or low risk for pregnancy.
We are entering an extremely exciting phase in the company’s life – delivering medical-grade features to our users at scale. We know there is a huge need and appetite for trustworthy products in this space and we are taking a lot of care to do this right. Our new investors are 100% committed to an approach that puts this respect for the individual’s unique experience and choices front and centre while leveraging the power of science and data to give people the reliable information they need to live their menstrual and reproductive lives to the fullest.
We are entering an extremely exciting phase in the company’s life – delivering medical-grade features to our users at scale. We know there is a huge need and appetite for trustworthy products in this space and we are taking a lot of care to do this right. Our new investors are 100% committed to an approach that puts this respect for the individual’s unique experience and choices front and centre while leveraging the power of science and data to give people the reliable information they need to live their menstrual and reproductive lives to the fullest.
Carrie Walter, co-CEO at Clue
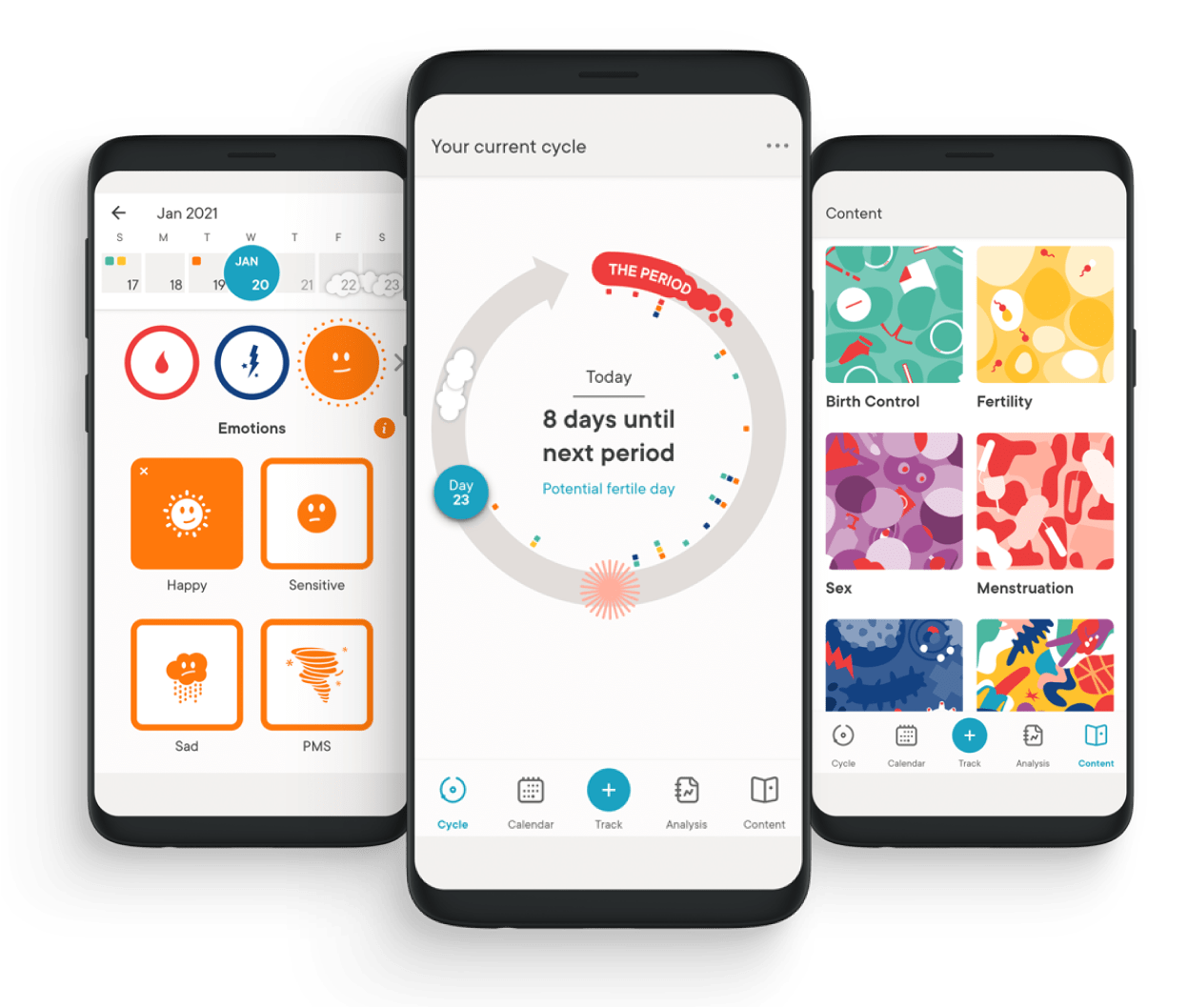
The Clue app
Clue has positioned itself as a category-defining product within femtech. They have built a friendly, user-centric product rooted in an evidence-based and medical-grade level of rigour with half the planet as potential customers over their lifetime.
Clue has positioned itself as a category-defining product within femtech. They have built a friendly, user-centric product rooted in an evidence-based and medical-grade level of rigour with half the planet as potential customers over their lifetime.
Colin Hanna, Partner, Balderton
When the FDA gave Clue clearance to offer this feature, the US regulator examined a Clue Birth Control clinical trial and subjected Clue’s quality management processes to extensive scrutiny. The clinical trial determined that Clue Birth Control’s algorithm is 92% effective with typical use (not always used according to instructions) and 97% effective with perfect use (always used exactly according to instructions). For comparison, the pill is 93% effective with typical use and 99.7% effective with perfect use. Condoms alone are 87% with typical use and 98% effective with perfect use.
Clue Birth Control is first launching in the USA where there is a strong need for better access to contraception. More than 19 million American women live in what is known as a ‘contraceptive desert‘, without reasonable access to local health care centres which offer a full range of contraceptive options. When Clue users were asked what they wanted in a contraception method, they said they wanted a tech-driven birth control option that was side-effect free, hormone free, easy to use, and evidence based. This was the impetus for the creation of Clue Birth Control.Clue will use the new funding to grow its team in Berlin and invest in building new products, as part of its vision to offer modes that support every part of the menstrual health journey, including from first period to pregnancy, post-partum, perimenopause and menopause. Clue also maintains a family-friendly work environment and benefits such as free weekly yoga, a free on-site therapist and a Flex Fridays policy to ensure their teams have time to enjoy all aspects of life, can put their health and wellbeing first, stay energised and maintain a sustainably high level of productivity.